NAVIGATIONNavigate Our Services
CTSI Navigation provides core support and resources to investigators performing translational research at The Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center (TLI)

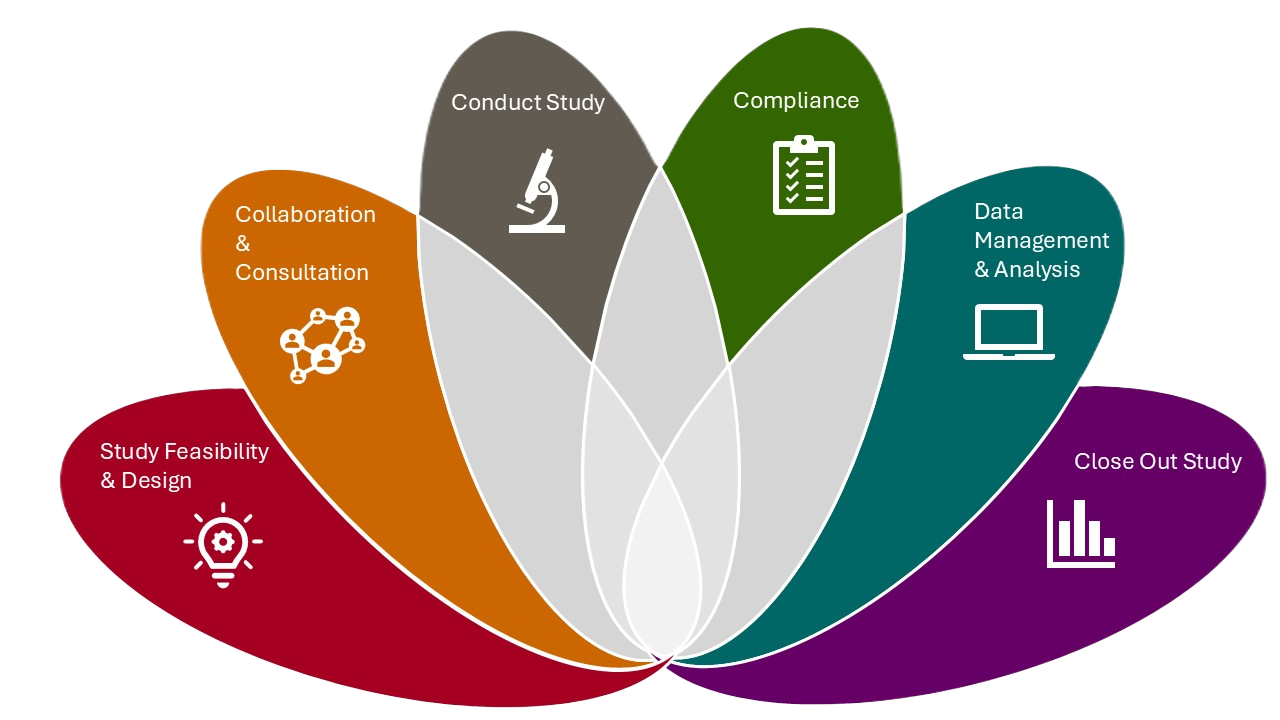
CTSI Navigation provides essential support and resources for researchers conducting translational studies at The Lundquist Institute for Biomedical Innovation at Harbor-UCLA Medical Center (TLI). From study design to closeout, our services streamline the research process, ensuring efficiency, compliance, and impactful results. To explore specific areas of support, click on the petals of the lotus symbol for more details.
Study Feasibility & Design
- Study Feasibility | Cohort Finding
- Biostatistics & Study Design
- Radiation Safety
Collaboration & Consultation
- Career development
- Find Collaborators
- Expert Consultations
- Community
- Special Populations
- Health Services & Outcomes
Conduct Study
- Investigational Drug Service
- Clinical Research Support
- Clinical and Translational Research Center (CTRC)
- Recruitment & Regulatory Support
- Animal Research & Biological Resources Center
- Department of Health Services (DHS) Research Request
Compliance
- Institutional review Board (IRB) Submission
- Project Number, Proposal, Review, Approval & Compliance Certification (PRACC), Budgets
- Grant Submission & Contract Negotiation
- Human Subjects Training
Data Management & Analysis
- Biostatistics
- Clinical Trials Management System (CTMS)
- REDCap Data Management
Close Out Study
- Sponsor | IRB Notification
- Record Retention Guidelines
- Media Outreach
- NIH Public Access Policy
- PubMed Central

